Caustic Soda
Caustic Soda
caustuc soda is a white compound that is soluble in water and is produced through the chemical decomposition of sodium chloride.
caustic soda is used in the production of soaps, shampoos and fatty substances because it has a great power in hydrolyzing fat molecules. caustic soda is also known as caustic soda and caustic soda, and its scientific name is sodium hydroxide.
caustic soda has a chemical composition that has a strong alkaline property and is considered as a pH regulator, and it can be used to neutralize acids and produce all kinds of salts.Physical characteristicsCaustic soda is a white solid and its melting point is 318 degrees Celsius.
It is very soluble in water and partially soluble in ethanol and methanol. There are different types of waterproofing in the form of 7 water with the formula NaOh.7H2O and 5 and 3 water, but the single water type has the most stable state.
Chemical propertiescaustic soda reacts with protonated acids and the product of this reaction is water and acid salt. For example, in case of reaction with hydrochloric acid, sodium chloride is produced. The reaction of caustic soda with acidic oxides such as sulfur dioxide is also very important because it is very important for desulfurization in petrochemical industries.
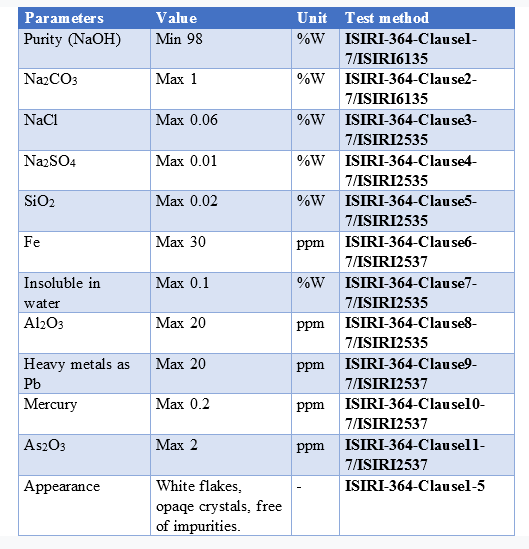
Caustic soda flakes analyses